The ionization energy of He+ is 19.6 x 10–18 J atom–1. The energy of the first stationary state (n = 1) of Li2+ is
-
4.41 x 10-16 J atom–1
-
-4.41 x 10-17 J atom–1
-
-2.2 x 10-15 J atom–1.
-
8.82 x 10-17 J atom–1.
B.
-4.41 x 10-17 J atom–1
Ionisation energy of He+
= 19.6 × 10-18 J
E1 (for H) × Z2= IE
E1 × 4 = – 19.6 × 10-18 J
E1 (for Li2+) = E1 for H × 9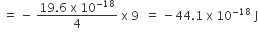
-4.41 x 10-17 J atom–1



