The formation of the oxide ion O2-(g) requires first an exothermic and then an endothermic step as shown below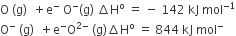
-
Oxygen is more electronegative
-
O- ion has comparatively larger size than oxygen atom
-
O- ion will tend to resist the addition of another electron
-
Oxygen has high electron affinity
C.
O- ion will tend to resist the addition of another electron

This process is unfavourable in the gas phase because the resulting increase in electron-electron repulsion overweights the stability gained by achieving the noble gas configuration.



