Question
For the reaction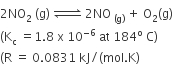
When Kp and Kc are compared at 184oC, it is found that
-
Kp is greater than Kc
-
Kp is less than Kc
-
Kp = Kc
-
Whether Kp is greater than, less than or equal to Kc depends upon the total gas pressure
Solution
A.
Kp is greater than Kc
Kp = Kc RT∆n n =1
Kp > Kc



