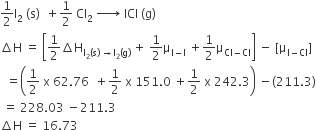The enthalpy changes for the following processes are listed below:
Cl2(g) = 2Cl(g), 242.3 kJ mol–1
I2(g) = 2I(g), 151.0 kJ mol–1
ICl(g) = I(g) + Cl(g), 211.3 kJ mol–1
I2(s) = I2(g), 62.76 kJ mol–1
Given that the standard states for iodine and chlorine are I2(s) and Cl2(g), the standard enthalpy of formation for ICl(g) is
-
–14.6 kJ mol–1
-
–16.8 kJ mol–1
-
+16.8 kJ mol–1
-
+244.8 kJ mol–1
C.
+16.8 kJ mol–1