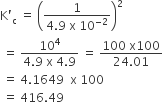Question
The equilibrium constant for the reaction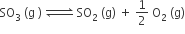
is Kc = 4.9 × 10–2. The value of Kc for the reaction'
2SO2 (g) +O2(g) ⇌ 2SO3 (g) will be
-
416
-
2.40 × 10–3
-
9.8 × 10–2
-
4.9 × 10–2
Solution
A.
416