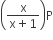
Phosphorus pentachloride dissociates as follows, in a closed reaction vessel,
PCl5 (g) ⇌ PCl3(g) + cl2(g)
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is x, the partial pressure of PCl3 will be
A.
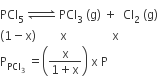
Phosphorus pentachloride dissociates as follows, in a closed reaction vessel,
PCl5 (g) ⇌ PCl3(g) + cl2(g)
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is x, the partial pressure of PCl3 will be

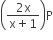

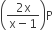
A.

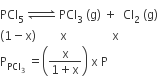
How pressure of a given sample of gas is related to absolute temperature at constant volume?
How is the pressure of a gas related to the number of molecules of the gas at constant temperature and volume?
What is standard (or normal) temperature and pressure (STP)?
What does SATP stand for? Define it.
What is the value of molar volume at STP?
What is standard molar volume?
What is the value of gas constant in SI units?
What is meant by aqueous tension?
Mock Test Series