Uncertainty in the position of an electron (mass = 9.1 × 10–31 kg) moving with a velocity 300 ms–1, accurate upto 0.001%, will be
-
19.2 × 10–2 m
-
5.76 × 10–2 m
-
1.92 × 10–2 m
-
3.84 × 10–2 m
C.
1.92 × 10–2 m
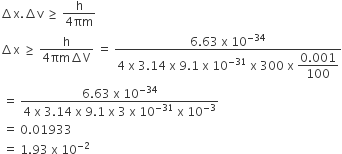
Uncertainty in the position of an electron (mass = 9.1 × 10–31 kg) moving with a velocity 300 ms–1, accurate upto 0.001%, will be
19.2 × 10–2 m
5.76 × 10–2 m
1.92 × 10–2 m
3.84 × 10–2 m
C.
1.92 × 10–2 m
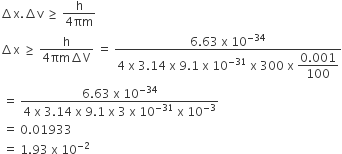
Name the particles which determine the mass of an element.
What are α-particles?
What are the fundamental particles present in a neutral atom having atomic number greater than 1?
Do protons and neutrons have identical mass?
When α-particles are sent through a thin metal foil, most of them go straight through the foil. What inference do you draw from it?
What did Rutherford's experiment on scattering of particles show for the first time?
What is Plum-Pudding model of the atom?
Are neutrons present in all atoms?
Mock Test Series