Sponsor Area
Equilibrium
Question
The pKa of a weak acid, HA, is 4.80. The pKb of a weak base, BOH, is 4.78. The pH of an aqueous solution of the corresponding salt, BA, will be
-
9.58
-
4.79
-
7.01
-
9.22
Solution
C.
7.01
It is a salt of weak acid and weak base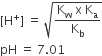
Some More Questions From Equilibrium Chapter
What is equilibrium?
Give two examples of physical equilibrium.
What kind of molecules in a liquid can evaporate?
Name the factors on which vapour pressure of any liquid depends.
Which measurable property becomes constant in water vapour equilibrium?
Give an example from daily life in which there is gas solution equilibrium?
A crystal of common salt of a given mass is kept in its aqueous solution. After 24 hours, its mass remains the same. Is the crystal in equilibrium with the solution?
Sponsor Area
Mock Test Series
Mock Test Series





