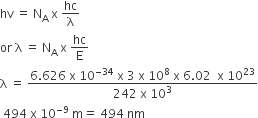The energy required to break one mole of Cl— Cl bonds in Cl2 is 242 kJ mol–. The longest wavelength of light capable of breaking a single Cl — Cl bond is
(c= 3 x 108 ms–1and NA = 6.02 x 1023 mol–1)
-
594 nm
-
640 nm
-
700 nm
-
494
D.
494
Energy, E = NA