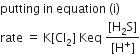Question
Consider the reaction:
Cl2(aq) + H2S(aq) → S (s) + 2H+ (aq) + 2Cl- (aq)
The rate equation for this reaction is
I. Cl2 + H2S → H+ +Cl- + Cl+ +HS-
II. H2S ⇌ H+ + HS- (fast equilibrium)
Cl2 + HS- → 2Cl- + H+ + S (slow)
-
II only
-
Both (I) and (II)
-
Neither (I) nor (II)
-
(I) only
Solution
D.
(I) only
For (A)
rate = K[Cl2] [H2S]
For (B)
rate = K[Cl2] [HS-] … (i)