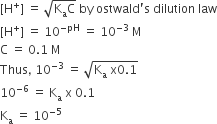Question
The pH of a 0.1 molar solution of the acid HQ is 3. The value of the ionisation constant, Ka of this acid is
-
3 x 10–1
-
1 x 10–3
-
1 x 10—5
-
1 x 10–7
Solution
C.
1 x 10—5
HQ = H+ + Q-