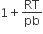Sponsor Area
States Of Matter
The compressibility factor for a real gas at high pressure is
C.
Vander Waal's equation for one mole of real gas is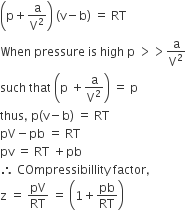
Some More Questions From States of Matter Chapter
What is absolute temperature?
What is the absolute zero temperature?
Can absolute zero temperature be attained for a gas?
How pressure of a given sample of gas is related to absolute temperature at constant volume?
How is the pressure of a gas related to the number of molecules of the gas at constant temperature and volume?
What is standard (or normal) temperature and pressure (STP)?
What does SATP stand for? Define it.
What is the value of molar volume at STP?
What is standard molar volume?
Sponsor Area
Mock Test Series
Mock Test Series