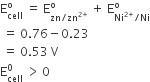The standard reduction potentials for Zn2+/ Zn, Ni2+/ Ni, and F2+/ Fe are –0.76, –0.23 and –0.44 V respectively. The reaction X + Y2+ → X 2+ + Y will be spontaneous when
-
X = Ni, Y = Fe
-
X = Ni, Y = Zn
-
X =Fe, Y= Zn
-
X = Zn, Y = Ni
D.
X = Zn, Y = Ni
X = Zn, Y = Ni
Zn + Ni2+ →Zn2+ + Ni