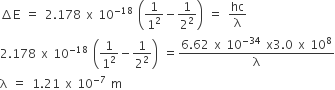Energy of an electron is given by
E =- 2.178 x 10-18 J 
Wavelength of light required to excite an electron in a hydrogen atom from level n =1 to n=2 will be (h=6.62 x 1034 Js and c = 3.0 x 108 ms-1)
-
1.214 x 10-7 m
-
2.816 x 10-7 m
-
6.500 x 10-7 m
-
8.500 x 10-7
A.
1.214 x 10-7 m