Question
For the reaction,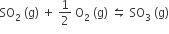
if Kp = Kc (RT)x where the symbol has usual meaning then the value of x is (assuming ideality)
-
-1
-
-1/2
-
1/2
-
1
Solution
B.
-1/2
For the given reaction, ∆ng = np-nR
where np = number of moles of products
nR = number of moles of reactants
Kp = Kc (RT)∆ng
∆ng = -1/2



