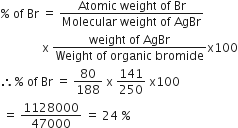In Carius method of estimation of halogens, 250 mg of an organic compound gave 141 mg of AgBr. The percentage of bromine in the compound is: (at. Mass Ag = 108; Br = 80)
-
24
-
36
-
48
-
60
A.
24
Weight of Organic compound = 250 mg
Weight of AgBr = 141 mg
therefore, According to the formula of % of bromine by Carius method