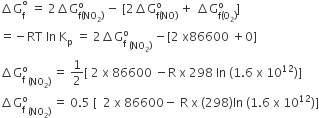The following reaction is performed at 298 K
2NO(g) + O2 (g) ⇌ 2NO2 (g)
The standard free energy of formation of NO (g) is 86.6 kJ/mol at 298 K. What is the standard free energy of formation of NO2 (g) at 298 K? (KP = 1.6 x 1012)
-
R (298) In (1.6 x 1012)-86600
-
86600 + R (298) In (1.6 x 1012)
-
86600 - In(1.6 x 1012)/R(298)
-
0.5[2 x 86600-R(298)In (1.6 x 1012)]
D.
0.5[2 x 86600-R(298)In (1.6 x 1012)]
For the given reaction,
2NO(g) + O2 (g) ⇌ 2NO2 (g)
Given ,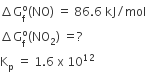
Now, we have,