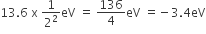Which of the following is the energy of a possible excited state of hydrogen?
-
+13.6 eV
-
-6.8 eV
-
-3.4 eV
-
+6.8 eV
C.
-3.4 eV
Since at n=1 the population of electrons is maximum i.e. at the ground state. So, maximum excitation will take place from n = 1 to n=2.
Hence, n=2 is the possible excited state,
Now, we have the formula for energy of H-atom
(En)H = 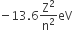
where Z = atomic number
Z for H-atom = 1
therefore, (En)H =