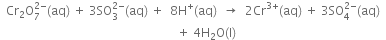With the net ionic equation for the reaction of potassium dichromate (VI), K2Cr2O7 with sodium sulphite, Na2SO3, in an acid solution to give chromium (III) ion and the sulphate ion.
(i) The skeleton ionic equation is
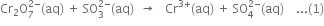
(ii) Write the O.N. of each atom and identify the atoms which undergo a change in O.N.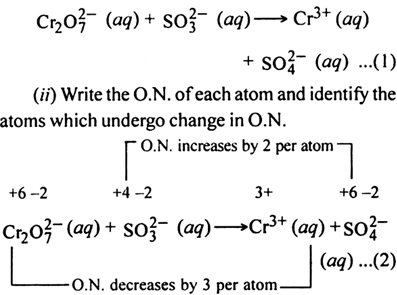
(iii) Calculate the total increase and decrease in O.N.
Since there are two Cr atoms on L.H.S. and only one on R.H.S., therefore multiply Cr3+on R.H.S. of (1) by 2 and thus a total decrease in O.N. of Cr is 2x3 = 6.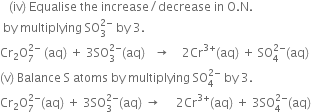
(vi) Balance O atoms by adding 4H2O molecules towards the R.H.S.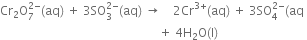
(vii) Balance H atoms by adding 8H ions on the L.H.S., since the reaction, occurs in the acidic medium.