Calculate the oxidation number of:
(i) Cr in dichromate ion
(ii) Mn in manganate ion
(iii) S in tetrathionate ion.
(i) Let the oxidation number of Cr in dichromate ion 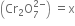
Writing the oxidation number of each atom at the top of its symbol
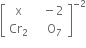
The algebraic sum of oxidation number of various atoms = 2
2(x) + 7(-2) = 0
or 2x - 14 = -2
2x = 12 x = +6
x = +6
(ii) Let the oxidation number of Mn in manganate ion 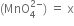
Writing the oxidation number of each atom at the top of its symbol
x -2
[Mn O4]2-
The algebraic sum of the oxidation number of various atoms = -2
x + 4(-2) = -2
x - 8 = -2
x = +6
(iii) Let the oxidation number of S in tetrathionate ion 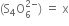
Writing the oxidation number of each atom at the top of its symbol.
x -2
[S4 O6]2-
The algebraic sum of oxidation number of various atoms = -2
4(x) + 6(-2) = -2
or 4x - 12 = -2
or 4x = 10
Therefore , x = 5/2.



