Discuss briefly the types of redox reactions. Give examples.
or
Discuss the following redox reactions.
(a) Combination reactions
(b) Decomposition reactions
(c) Displacement reactions
(d) Disproportionation reactions.
Give one example in each case.
Redox reactions have been divided into the following classes.
(a) Combination reactions. In such reactions, two or more elements combine chemically to form compounds. The chemical reactions involving the participation of oxygen (combustion reactions) and also few others which involve a change in the oxidation number of some atoms are the examples of such redox reactions.
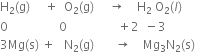
(b) Decomposition reactions: In such reactions, a compound either of its own or upon heating decomposes to produce two or more components. Out of such components, at least one component must be in the elemental state. For example,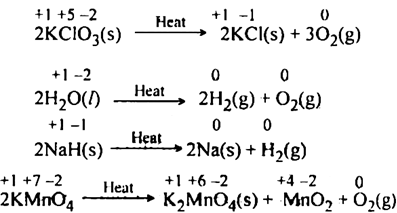
There are also some decomposition reactions which are not redox reactions in nature because in such reactions there is no change in Oxidation number of the reactant species. ![]()
(c) Displacement reactions: In such reactions, a weak atom/ion present in a compound gets replaced by strong atom/ion of another element. such as,![<pre>uncaught exception: <b>Http Error #503</b><br /><br />in file: /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php line 61<br />#0 [internal function]: com_wiris_plugin_impl_HttpImpl_0(Object(com_wiris_plugin_impl_HttpImpl), NULL, 'http://www.wiri...', 'Http Error #503')
#1 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/php/Boot.class.php(769): call_user_func_array('com_wiris_plugi...', Array)
#2 [internal function]: _hx_lambda->execute('Http Error #503')
#3 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(532): call_user_func_array(Array, Array)
#4 [internal function]: haxe_Http_5(true, Object(com_wiris_plugin_impl_HttpImpl), Object(com_wiris_plugin_impl_HttpImpl), Array, Object(haxe_io_BytesOutput), true, 'Http Error #503')
#5 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/php/Boot.class.php(769): call_user_func_array('haxe_Http_5', Array)
#6 [internal function]: _hx_lambda->execute('Http Error #503')
#7 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php(27): call_user_func_array(Array, Array)
#8 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(444): com_wiris_plugin_impl_HttpImpl->onError('Http Error #503')
#9 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(458): haxe_Http->customRequest(true, Object(haxe_io_BytesOutput), NULL, NULL)
#10 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php(40): haxe_Http->request(true)
#11 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/TextServiceImpl.class.php(80): com_wiris_plugin_impl_HttpImpl->request(true)
#12 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/service.php(19): com_wiris_plugin_impl_TextServiceImpl->service('mathml2accessib...', Array)
#13 {main}</pre>](/application/zrc/images/qvar/CHEN11091214-4.png)
In this reaction, A has replaced B from the compound BC. These reactions are of two types.
(i) Metal displacement reactions. In such reactions, a metal present in a compound gets displaced by a metal in the uncombined state provided it occupies a position higher in the activity series. For example,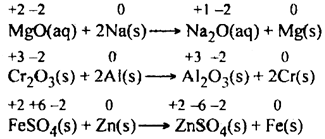
(ii) Non-metal displacement reactions Such reactions include hydrogen displacement and very few occurring reactions involving oxygen displacement, All alkali metals and few alkaline earth Sr metals(Ca, Sr and Ba) known to be very good reducing agents will displace hydrogen from cold water.![]()

(d) Disproportionation reaction: Such reactions, the same substance acts as oxidant as well as reductant simultaneously i.e. a Chemical reaction in which same species undergoes Oxidation (oxidation number increases) as well as reduction (oxidation number decreases);
For example:
(i) Hydrogen peroxide (H2O2) is quite unstable and undergoes? disproportionation.![]()
Oxygen of H2O=2 in -1 oxidation state is converted to , (0) oxidation state as well as (-2) oxidation state.
(ii) In alkaline solution, phosphorus (P4) undergoes disproportionation.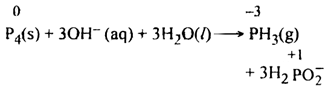
In the above reaction oxidation state of P4(0) is converted to (-3) in PH3 and to (+1) in 



