Question
Write correctly balanced equation for the following reaction using half reactions: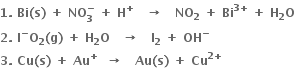
Solution
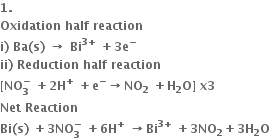

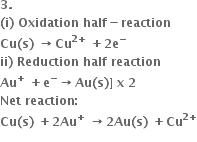
Sponsor Area
Write correctly balanced equation for the following reaction using half reactions: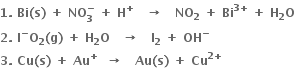
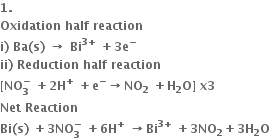

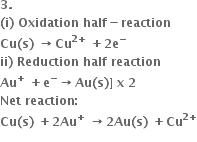
Can oxidation occur without reduction?
What are redox reactions?
What are indirect redox reactions?
Write formulas for the following compounds:
(a) Mercury (II) chloride
(b) Nickel (II) sulphate
(c) Tin (IV) oxide
(d) Thallium (I) sulphate
(e) Iron (III) sulphate
(f) Chromium (III) oxide
Mock Test Series