Question
Identify the oxidant and the reductant in the following reactions: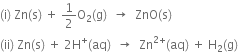
Solution
In reaction (i) zinc donates electrons to O to give zinc and oxide ions. Therefore, Zn acts as a reductant while oxygen acts as an oxidant.
In reaction (ii) Zn transfers its electrons to H+ and therefore, zinc acts as a reductant and H+ acts as an oxidant.



