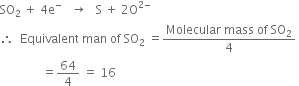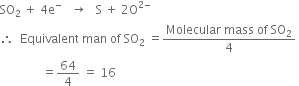Question
What is the equivalent mass of the oxidation agent in the reaction
Solution
In this reaction, SO2 oxidises H2S, therefore, SO2 acts as oxidising agent. During oxidation, four electrons are lost i.e.