Sponsor Area
Redox Reactions
Question
Write the following redox reaction using half equation:

Mention:
(i) Which reactant is oxidised? To what?
(ii) Which reactant is the oxidiser?
(iii) Which reactant is reduced? To what?
(iv) Which reactant is the reducer?
Solution
Oxidation half-reaction:
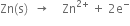
Reduction half-reaction:

Here Zn is oxidised to Zn
i) Zn is oxidised to Zn2+,
ii)Pb2+ is reduced to Pb.
iii) Pb2+ is the oxidiser and
iv) Zn is the reducer.
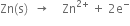
Reduction half-reaction:

Here Zn is oxidised to Zn
i) Zn is oxidised to Zn2+,
ii)Pb2+ is reduced to Pb.
iii) Pb2+ is the oxidiser and
iv) Zn is the reducer.
Some More Questions From Redox Reactions Chapter
What is oxidation (electronic concept)?
What is reduction (electronic concept)?
What is reducing agent (electronic concept)?
What is an oxidising agent (electronic concept)?
Can oxidation occur without reduction?
What are redox reactions?
What are direct redox reactions? Give one example.
What are indirect redox reactions?
Sponsor Area
Mock Test Series
Mock Test Series





