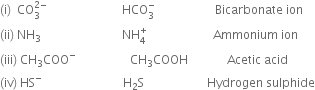Question
State the formula and name of conjugate acid of the following bases:
(i) ![]() (ii) NH3
(ii) NH3
(iii) CH3COO– (iv) HS–
Solution
Given Species Conjugate acid Name