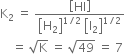What are the characteristics of equilibrium constant?
(i) The equilibrium constant has a definite value for every chemical reaction at a given temperature. However, it varies with the change in temperature.
(ii) Its value is not influenced by the change in the concentration of reactants and products.
(iii) It is not affected by the presence of a catalyst.
(iv) The equilibrium constant for the forward reaction is the inverse of the equilibrium constant for the backward reaction.
For ![]()
![]()
and for
![]()
![]()
Clearly
![]()
(v) The value of K tells us the extent to which the forward or backward reaction has taken place. The Greater value of Kc and Kp means that the reaction has proceeded to a greater extent in the forward direction.
(vi) The value of K changes if the coefficient of various species in the equation representing equilibrium are multiplied by the same number.
For example,
![]()
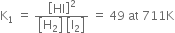
However, if we write ![]()
Then