Question
What do you mean by the standard enthalpy of combustion?
Solution
Standard enthalpy of combustion is defined as the enthalpy per mole of the substance, all the reactants and products being in their standard states i.e. at 298K and 1 bar pressure. It is denoted by ∆CH°.
(i) Combustion of butane:
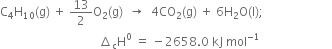
(ii) Combustion of glucose:
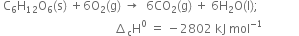
(i) Combustion of butane:
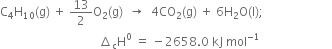
(ii) Combustion of glucose:



