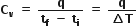What do you understand by:
(i) Heat capacity of a substance
(ii) Heat capacity at constant volume
(iii) Heat capacity at constant pressure?
(i) Heat capacity: The heat capacity (C) of a sample of a substance is the quantity of heat needed to raise its temperature by 1°C (or one kelvin).
(ii) Heat capacity at constant volume: The heat supplied to a system to raise its temperature through 1°C keeping the volume of the system constant is called heat capacity at constant volume.
(iii) Heat capacity at constant pressure: The heat supplied to a system to raise its temperature through 1 °C keeping the external pressure constant is called heat capacity at constant pressure.
If q is the amount of heat supplied to a sample and as a result, if the temperature of the sample changes from initial temperature to a final temperature tf, then the heat capacity is given by