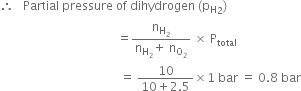A mixture of dihydrogen and dioxygen at one bar pressure contains 20% by weight of dihydrogen. Calculate the partial pressure of dihydrogen.
Since the mixture H2 and O2 contains 20% by weight of hydrogen, therefore if H2 = 20g, then O2 = 100 - 20 = 80 g![]() No. of moles of dihydrogen (H2)
No. of moles of dihydrogen (H2)
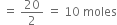
No. of moles of dioxygen (O2) = ![]()
Partial pressure of a gas = mole fraction of the gas X total pressure,