A gas cylinder containing cooking gas can withstand a pressure of 14.9 bar. The pressure gauge of the cylinder indicates 12 bar at 27°C. Due to sudden fire in the building, the temperature starts rising. At what temperature will the cylinder explode?
Since the gas is confirmed in a cylinder, its volume will remain constant.
From the given data,
![]()
Applying pressure temperature law,
![]()
![]()
Substituting the values in the above equation,
![]()
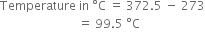
Hence the cylinder will at ![]()



