The electron gain enthalpy of chlorine is 3.7 eV per atom. How much enthalpy in kJ is released when 1g of chlorine is converted completely of Cl- ion in the gaseous state?
We know,
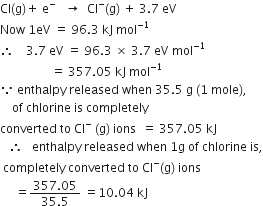
The electron gain enthalpy of chlorine is 3.7 eV per atom. How much enthalpy in kJ is released when 1g of chlorine is converted completely of Cl- ion in the gaseous state?
We know,
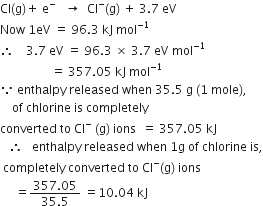
How many elements are placed in each period of the p-block?
What is the number of groups in: (i) p-block (ii) d-block?
How many elements are present in:
(i) second period
(ii) fourth period
(iii) sixth period?
What is the name given to the s-block elements?
To which block the element with outer electronic configuration 4s23d10 belongs?
Why are there 10 elements in each series of d-block?
In terms of electronic configuration,
what is common in a given period and group?
Give the general electronic configuration of
(i) Transition elements (ii) Inner transition elements.
Name the group and period to which an element with Z = 15 belongs ?
What are the advantages of periodic classification of elements?
Mock Test Series