Question
Explain which of the following will have the most negative electron gain enthalpy and which the least negative: P, S, CI, F.
Solution
The electronic configuration of P, S, E and CI can be represented as,
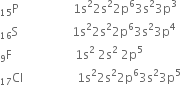
Electron gain enthalpy generally becomes more negative across a period as we move from left to right. Within a group, electron gain enthalpy becomes less negative down a group. However, adding an electron to the 2p orbital leads to greater repulsion than adding an electron to the larger 3p orbital. Hence the element with most negative electron gain enthalpy is chlorine ; the one with the least negative electron gain enthalpy is phosphorus.
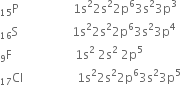
Electron gain enthalpy generally becomes more negative across a period as we move from left to right. Within a group, electron gain enthalpy becomes less negative down a group. However, adding an electron to the 2p orbital leads to greater repulsion than adding an electron to the larger 3p orbital. Hence the element with most negative electron gain enthalpy is chlorine ; the one with the least negative electron gain enthalpy is phosphorus.



