What do you mean by covalent radius?
rcov = 1/2 [Internuclear distance between two bonded atoms]
For example, for hydrogen molecule, the internuclear distance between two hydrogen atoms is 74 pm.
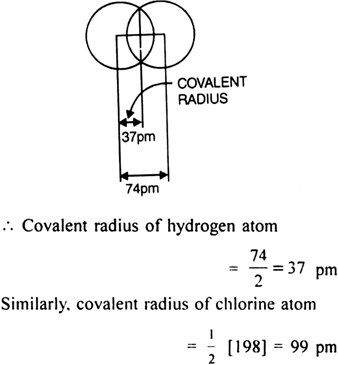
where 198 pm = Internuclear distance between two chlorine atoms (Cl—Cl) in a molecule of Cl2.
In case of heteronuclear molecules (containing different atoms) such as HCl, the covalent radius may be defined as: “The difference of the distance between the nuclei of two bonded atoms and covalent radius of one of the atoms in a heteronuclear molecule.”
Covalent radius = [Internuclear distance of two bonded atoms] -[Covalent radius of one of the two atoms in heteronuclear molecules]
For example,
Covalent radius of H-atom (in HCl)
= Internuclear distance of HCl - covalent radius of Cl atom
= 136 pm - 99 pm = 37 pm.



