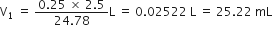If the density of methanol is 0·793 kg L–1, what is its volume needed for making 2·5L of its 0·25 M solution?
Molar mass of methanol (CH3OH)
= 12 + 4 x 1 + 16 = 32g mol-1
= 0.032 kg mol-1![]() Molarity of the given solution
Molarity of the given solution
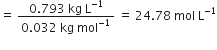
Applying the molarity equation,
(Given solution) 

or