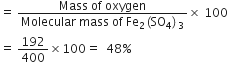Fe2(SO4)3 is the empirical formula of a crystalline compound of iron. It is used in water and sewage treatment for the removal of suspended impurities. Calculate the mass percentage of iron, sulphur and oxygen in the compound.
Molecular mass of 
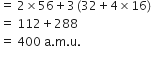
Percentage of iron

Percentage of sulphur
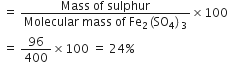
Percentage of oxygen