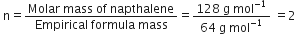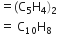Question
Naphthalene (moth balls) contains 93·71% carbon and 6·29% hydrogen. If its molar mass is 128 g mol–1, calculate its molecular formula.
Solution
(i) To calculate the empirical formula:
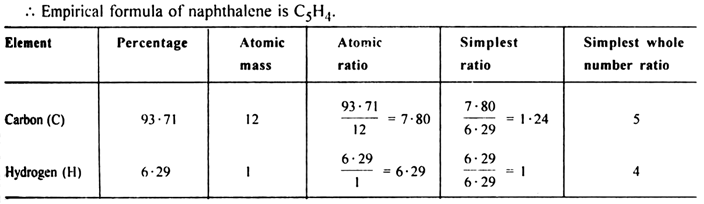
(ii) To calculate the molecular formula:
Empirical formual mass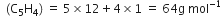
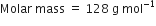 [given]
[given]
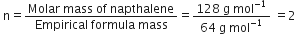
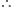 Molecular formula of napthalene =
Molecular formula of napthalene = 

(ii) To calculate the molecular formula:
Empirical formual mass
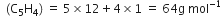
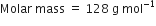 [given]
[given]