Discuss the chemistry of Lassaigne's test.
Nitrogen, sulphur, halogens and phosphorus present in an organic compound are detected by 'Lassaigne's test'. The elements present in the compound are converted from the covalent form into the ionic form by fusing the compound with sodium metal.
Reactions involved during fusion.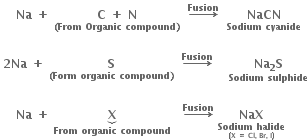
Cyanide, sulphide and halide of sodium so formed in sodium fusion are extracted from the fused mass by boiling it with distilled water. This extract is known as sodium fusion extract.



