How will you explain the tetracovalency of carbon?
The atomic number of carbon is 6 and its electronic configuration is 2, 4. In other words, carbon has four electrons in the valence shell and thus needs four more electrons to complete its octet. Therefore carbon is tetravalent. Since carbon atom has high ionisation energy and moderate electron affinity, therefore it is very difficult for carbon to either lose or gain four electrons to achieve the nearest inert gas configuration. Consequently, carbon always combines with other atoms by mutual sharing of electrons and thus forms covalent bonds. Thus, carbon is always tetravalent i.e. it forms four covalent bonds with other atoms.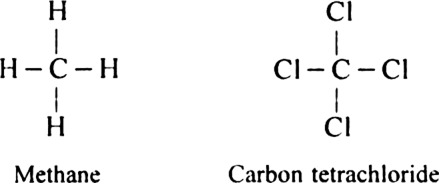
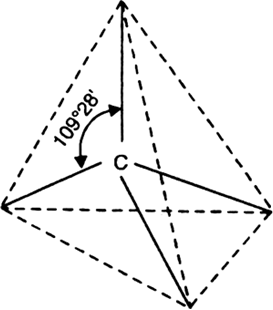
According to Le Bel and van’t Hoff, the four bonds of a carbon atom are directed towards the four corners of a regular tetrahedron. The carbon atom lies at the centre of a regular tetrahedron and the angle between any two adjacent bonds is 109° – 28' or 109.5°. This arrangement of carbon atom is regarded as a tetrahedral model or carbon or space model.