Discuss briefly the IUPAC and common names of few important aliphatic organic families.
(i) Mono halogen derivatives: These are halogen derivatives of alkanes.
General formula. CnH2n+1 – X or R–X where X is Cl, Br or 1.
Examples: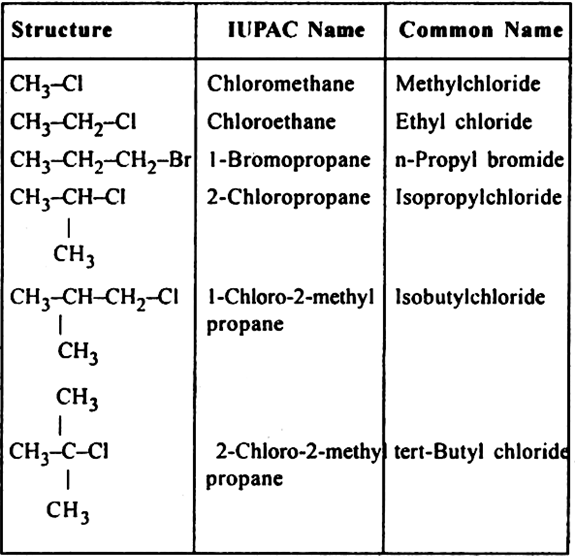
(ii) Polyhalogen derivatives: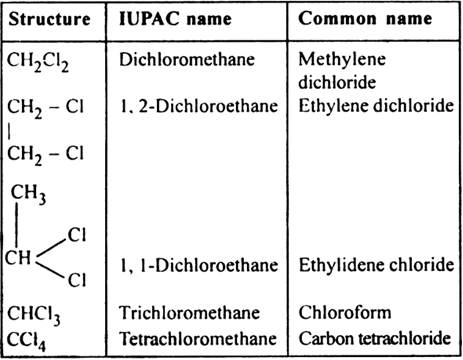
2. Alcohols or alkanols (suffix-ol)
(i) Monohydric alcohols or alkanols.
General formula CnH2n+1 – OH
Functional group –OH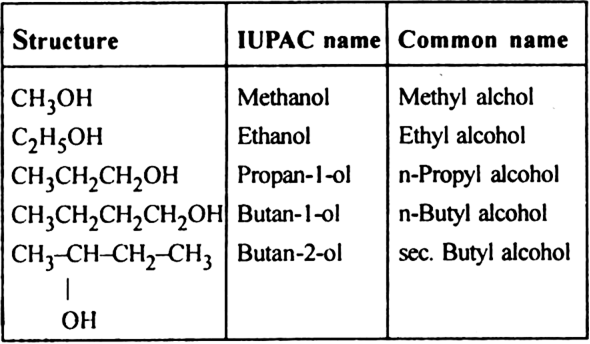
(ii) Dihydric alcohols or Alkanediols (suffix-diol)
General formula: CnH2n(OH)2
Two–OH groups are linked to two different carbon atoms.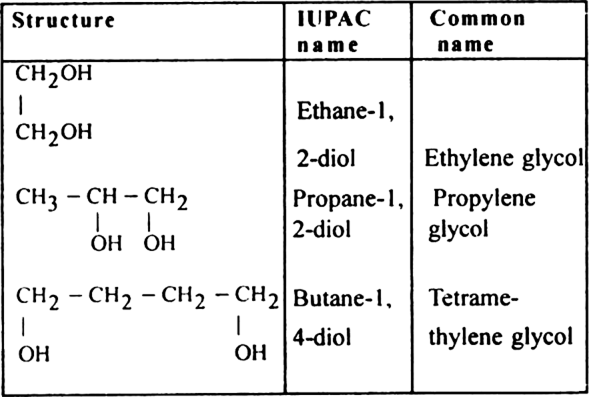
iii) Trihydric alcohols or alkane triols (suffix – triol)
General formula: CnH2n–1 (OH)3
Three –OH groups are linked to three different carbon atoms.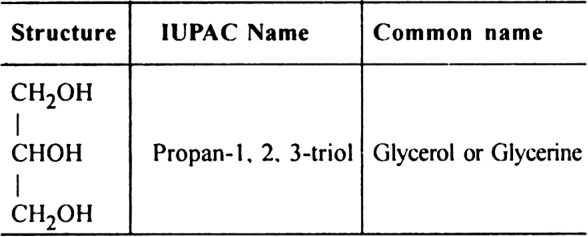
3. Ethers or alkoxy alkanes (prefi-alkoxy) General formula: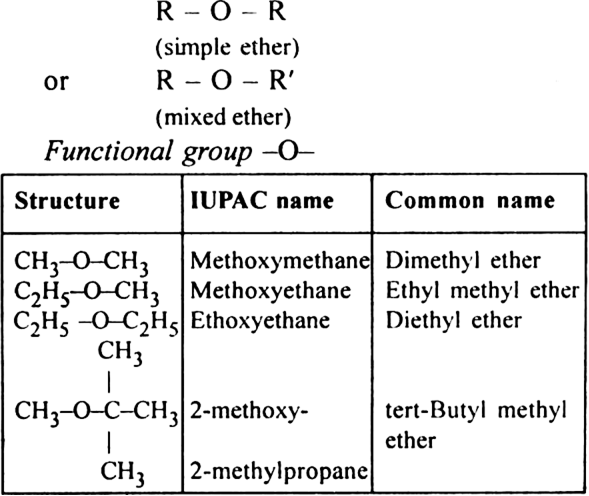
4. Aldehydes or Alkanals (suffix-al).
General formula CnH2n+1 – CHO or R–CHO
Functional group –CHO
Structure | IUPAC name | Common name |
HCHO | Methanal | Formaldehyde |
ch3cho | Ethanal | Acetaldehyde |
CH3CH2CHO | Propanal | Propionaldehyde |
CH3CH2CH2CHO | Bulanal n-Butyraldehyde |
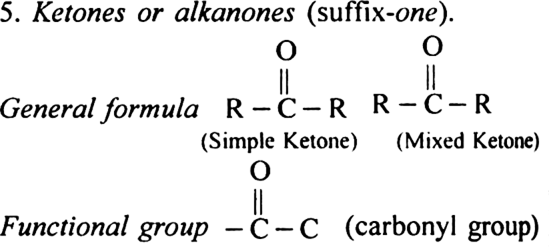
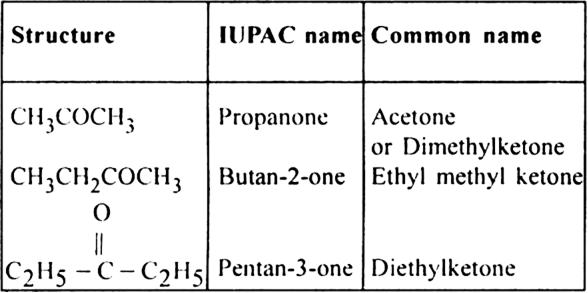
6. (i) Monocarboxylic acids or Alkanoic acids (suffix-oic acid).
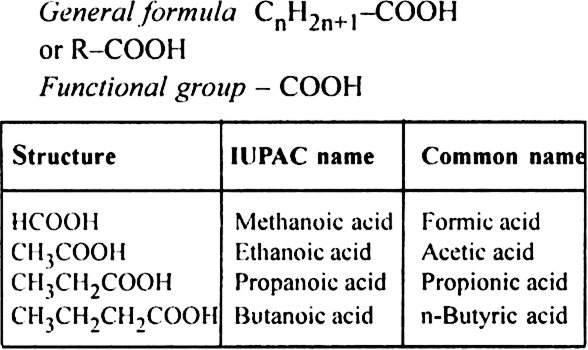
(ii) Dicarboxylic acid or Alkanedioic acid.
General formula C2H2n (COOH)2
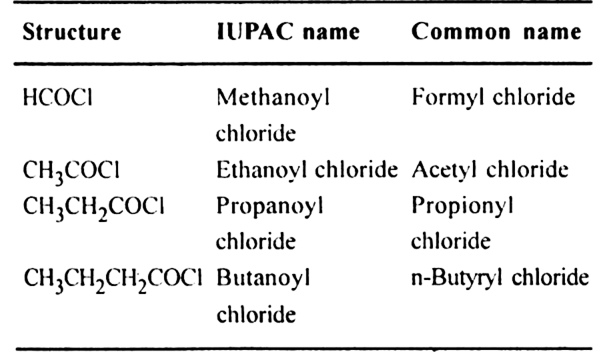
7. Acyl halides or Alkanoyl halide (suffix – oyl chloride)
General formula RCOCl;
Functional group –COCl
8. Acid amides or Alkanamides (suffix – amide).
General formula RCONH2;
Functional group –CONH2
Structure | IUPAC name | Common name |
HCONH2 | Methanamide | Formamide |
CH3CONH2 | Ethanamide | Acetamide |
CH3CH2CONH2 | Proponamide | Propionamide |
CH3CH2CH2CONH2 | Butanamide | n-Butyramide |
9. Esters or AIkyI alkanoate (suffix-oate).
General formula RCOOR or RCOOR'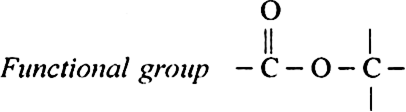
Structure | IUPAC name | Common name |
HCOOCH3 | Methyl methanoate | Methyl formate |
HCOOC2H5 | Ethyl methanoate | Ethyl formate |
CH3COOCH3 | Methyl ethanoate | Methyl acetate |
CH3COOC2H5 | Ethyl ethanoate | Ethyl acetate |
10. Acid anhydrides or Alkanoic anhydrides (suffix –oic anhydride). General formula
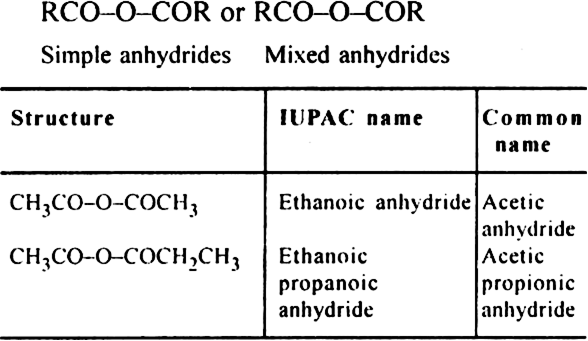
11. Primary amines (1°) or Alkanamines (suffix amine).
General formula R – NH2
Functional group – NH2 (Amino group)
The common name of an amine is always written as one word.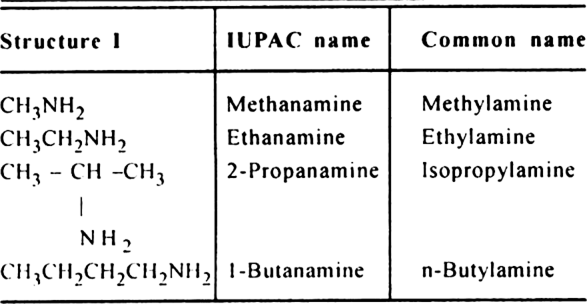
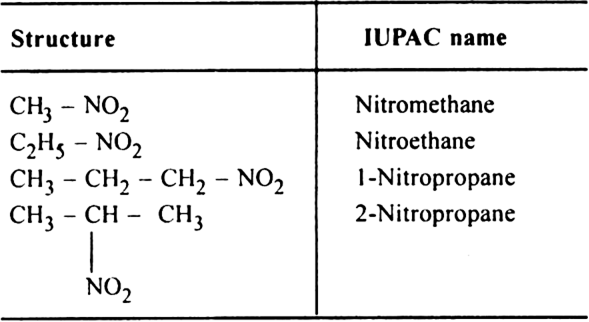
12. Nitroalkanes or nitro paraffines.
General formula R – NO2
Functional group –NO2 (called nitro group)
Nitro compounds are named under IUPAC system only.