Question
Contrast the structure and properties of CO2 and SiO2.
Solution
| Carbon dioxide(CO2) | Silica (SiO2) |
| 1. It is a monomeric linear gaseous molecule as O =C = O | 1. It is a solid network having a three-dimensional structure in which each silicon atom is tetrahedrally surrounded by four oxygen atoms. |
| 2. It is soluble in water. | 2. It is insoluble in water. |
3. On reduction with coke (carbon), it forms carbon monoxide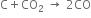 |
3. On reduction with carbon in an electric furnace, it produces silicon carbide.  |



