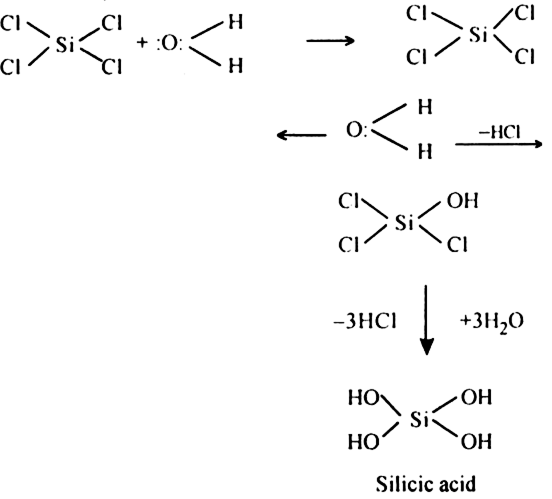
Explain why silicon tetrachloride is hydrolyzed but carbon tetrachloride is not hydrolysed.
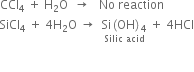
In this reaction, all the four chlorine atoms of SiCl4 are replaced by four OH groups.
Sponsor Area
Explain why silicon tetrachloride is hydrolyzed but carbon tetrachloride is not hydrolysed.

Which element of group 13 forms amphoteric hydroxide?
Which element of group 13 forms the most stable +1 oxidation state?
What is the nature of boron trifluoride?
What is the structure of diborane?
Can borax bead test be performed for Ca2+ ion?
Which is the hardest compound of boron?
What is inorganic benzene? Write its formula.
Name a hydride of boron which is used as reducing agent in organic chemistry.
Sponsor Area
Mock Test Series