Question
Explain structures of diborane. Write the structures of diborane and explain the nature of bonding in it ?
Solution
Electronic configuration of boron (Z=5) in the excited state is 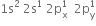 . There are three half filled orbitals in its valence shell. If each boron atom in B2H6 forms three covalent bonds, 14 electrons are required (six B-H bonds and one B– B bond). But there are only 12 electrons (six from two boron atoms and six from hydrogen atoms). So B2H6molecule is short of 2 electrons. Therefore, it cannot have a structure similar to that of C2H6 (ethane).
. There are three half filled orbitals in its valence shell. If each boron atom in B2H6 forms three covalent bonds, 14 electrons are required (six B-H bonds and one B– B bond). But there are only 12 electrons (six from two boron atoms and six from hydrogen atoms). So B2H6molecule is short of 2 electrons. Therefore, it cannot have a structure similar to that of C2H6 (ethane).
The electron diffraction studies have shown bridged structure for diborane.
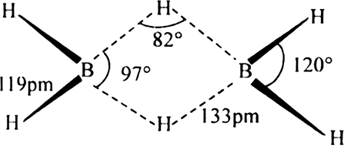
In this structure, the four terminal hydrogen atoms (shown by thick lines) and two boron atoms lie in one plane while other two hydrogen atoms, one lying above and the other lying below this plane and hence are called bridge hydrogens. The above structure of diborane depicts that there are two types of hydrogen atoms. Four of them are of one type which is used in making four normal covalent bonds (two centre electron pair bonds) with boron. The remaining two form bridges between two boron atoms through three centre electron pair bonds. Three centre electron pair bond is a bond involving three atoms and only two electrons.
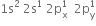 . There are three half filled orbitals in its valence shell. If each boron atom in B2H6 forms three covalent bonds, 14 electrons are required (six B-H bonds and one B– B bond). But there are only 12 electrons (six from two boron atoms and six from hydrogen atoms). So B2H6molecule is short of 2 electrons. Therefore, it cannot have a structure similar to that of C2H6 (ethane).
. There are three half filled orbitals in its valence shell. If each boron atom in B2H6 forms three covalent bonds, 14 electrons are required (six B-H bonds and one B– B bond). But there are only 12 electrons (six from two boron atoms and six from hydrogen atoms). So B2H6molecule is short of 2 electrons. Therefore, it cannot have a structure similar to that of C2H6 (ethane).The electron diffraction studies have shown bridged structure for diborane.

In this structure, the four terminal hydrogen atoms (shown by thick lines) and two boron atoms lie in one plane while other two hydrogen atoms, one lying above and the other lying below this plane and hence are called bridge hydrogens. The above structure of diborane depicts that there are two types of hydrogen atoms. Four of them are of one type which is used in making four normal covalent bonds (two centre electron pair bonds) with boron. The remaining two form bridges between two boron atoms through three centre electron pair bonds. Three centre electron pair bond is a bond involving three atoms and only two electrons.



