Suggest a reason why the B - F bond lengths in BF3 (130 pm) and  (143 pm) differ?
(143 pm) differ?
Or
Why B - F bond length in BF3 is smaller than the expected value?
In BF3, boron is sp2 hybridised and therefore BF3 is a planar molecule. It has a vacant 2p-orbital. F-atom has three lone pairs of electrons. In BF3 molecule, one 2p-orbital of fluorine atom overlaps sidewise with empty 2p-orbtial of boron to form  back bonding (back donation) in which the lone pair is transferred from F to B as shown.
back bonding (back donation) in which the lone pair is transferred from F to B as shown.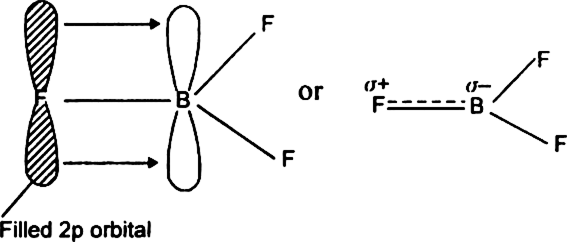
As a result of this back bonding (or black donation), the B-F bond acquires some double bond character.
On the other hand in 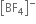 ion, boron is sp3 hybridised and therefore
ion, boron is sp3 hybridised and therefore 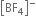 is a tetrahedral molecule. B in
is a tetrahedral molecule. B in 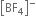 ion does not have vacant p-orbital available to accept the electrons donated by the F atom. Hence
ion does not have vacant p-orbital available to accept the electrons donated by the F atom. Hence 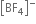 ion, B -F is a purely single bond. Since double bonds are shorter than single bonds, therefore B-F bond length in BF3 is shorter (130 pm) than B-F bond length (143 pm) in [BF4]–.
ion, B -F is a purely single bond. Since double bonds are shorter than single bonds, therefore B-F bond length in BF3 is shorter (130 pm) than B-F bond length (143 pm) in [BF4]–.



