Question
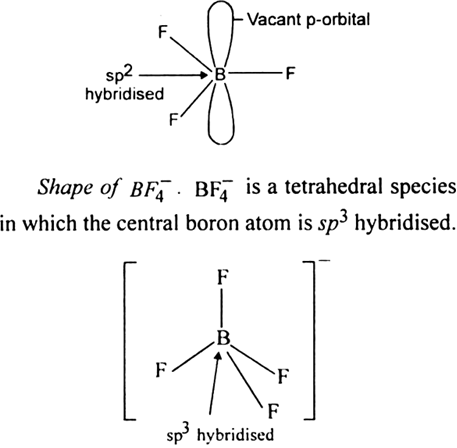
Describe the shapes of BF3 and  . Assign the hybridization of boron in these species ?
. Assign the hybridization of boron in these species ?
Solution
The shape of BF3 is a planar molecule in which the central boron atom is sp2 hybridised. A sp2 hybridised boron atom has a vacant p-orbital.