Sponsor Area
The P-Block Elements
Boron is unable to form 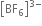 ion. Explain ?
ion. Explain ?
 ion is not formed due to non-availability of d-orbitals in boron. Therefore, the maximum covalence of boron can not exceed 4.
ion is not formed due to non-availability of d-orbitals in boron. Therefore, the maximum covalence of boron can not exceed 4.
Some More Questions From The p-Block Elements Chapter
Which element of group 13 forms the most stable +1 oxidation state?
What is the nature of boron trifluoride?
What is the structure of diborane?
Can borax bead test be performed for Ca2+ ion?
Which is the hardest compound of boron?
What is inorganic benzene? Write its formula.
Name a hydride of boron which is used as reducing agent in organic chemistry.
Does a molecule of BH3 exist?
Sponsor Area
Mock Test Series
Mock Test Series





