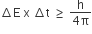With respect to the conformers of ethane, which of the following statements is true?
-
Bond angle remains same but bond length changes
-
Bond angle changes but bond length remains same
-
Both bond angle and bond length change
-
Both bond angles and bond length remains same
D.
Both bond angles and bond length remains same
There is no change in bond angles and bond lengths in the conformations of ethane. There is only change in dihedral angle.