A gas such as carbon monoxide would be most likely to obey the ideal gas law at
-
high temperatures and low pressures
-
low temperatures and high pressures
-
high temperatures and high pressures
-
low temperatures and low pressures
A.
high temperatures and low pressures
Real gases show ideal gas behaviour at high temperatures and low pressures.
PV = nRT






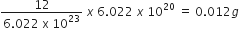
 is
is



