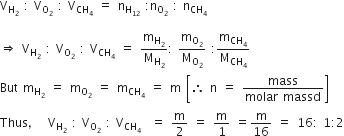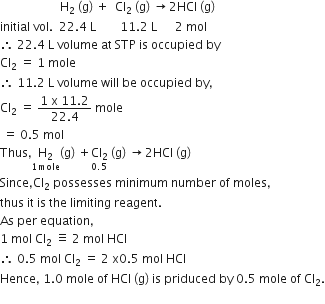What is the maximum number of orbitals that can be identified with the following quantum numbers?
n=3, l =1, m1 = 0
-
1
-
2
-
3
-
4
A.
1
The value of n=3 and l =1 suggest that it is a 3p orbital while the value of m1 = 0 [magnetic quantum number] shows that the given 3p orbital is 3pz in nature.
Hence, the maximum number of orbitals identified by the given quantum number is only 1, i.e. 3pz.