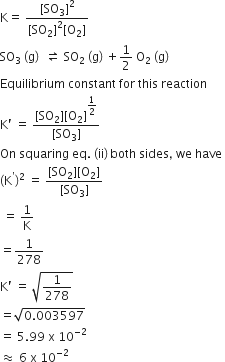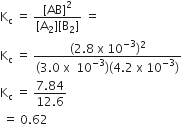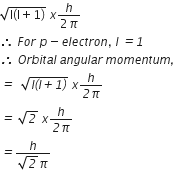Give that the equilibrium constant for the reaction,
2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
has a value of 278 at a particular temperature. What is the value of the equilibrium constant for the following reaction t the same temperature?
SO3 (g) ⇌ SO2 (g) +1/2 O2 (g)
-
1.8 x 10-3
-
3.6 x 10-3
-
6.0 x 10-2
-
1.3 x 10-5
C.
6.0 x 10-2
2SO2 (g) +O2 (g) ⇌ 2SO3 (g)
Equilibrium constant for this reaction,